Clinical Research Organization (CRO) and Regulatory Affairs Consultancy in Latin America
We lead innovation that changes lives
Clinical Research – CRO
- We help you to develop any stage
of a clinical trial - Regulatory and legal representation
Pharmaceutical
- Regulatory consulting
- Implementing of 24/7 pharmacovigilance systems
Cosmetic
- Sanitary registration of products
- Regulatory consulting
An expert partner in Latin America
We provide services to the pharmaceutical and biotechnology industry for the development of clinical trials from Phase I to IV. Also, we guide companies through the challenges related to regulatory issues within the pharmaceutical market.
Based in Chile, we have conducted studies throughout Latin America for the most important multinationals with a 100% committed team, senior researchers, exhaustive control of all processes and strategic alliances throughout the region.
Our experience and local knowledge will benefit your project, with cost-effective and high-quality management.
Life-changing research
Experience in clinical trials (chronic pain, infectious diseases, diabetes, hematology, oncology, respiratory/pulmonary, reumathology) and vaccine studies (COVID-19, Influenza).
We put you ahead of your competition with our expertise and partnerships in North and South America.

Working together to overcome the pandemic
Largest COVID-19 vaccine trial in Chile with
3,500 volunteers
First COVID-19
Clinical trial in Chile with inhaled vaccine
We are a Contract Research Organization (CRO) and regulatory consultancy dedicated to providing services to the pharmaceutical and biotechnology industry
for the development of clinical trials from Phase I to IV.
Our solutions and capabilities
What we do in regulatory consulting
- Representation as Technical Direction of companies to the regulatory authority of Chile (Institute of Public Health)
- Regulatory affairs intelligence
- Health registration – Bioequivalents
- Management of importation with the Chilean regulatory authority (Institute of Public Health).
- Implementation of quality management systems (GMP, GLP)
- Implementation of 24/7 pharmacovigilance systems

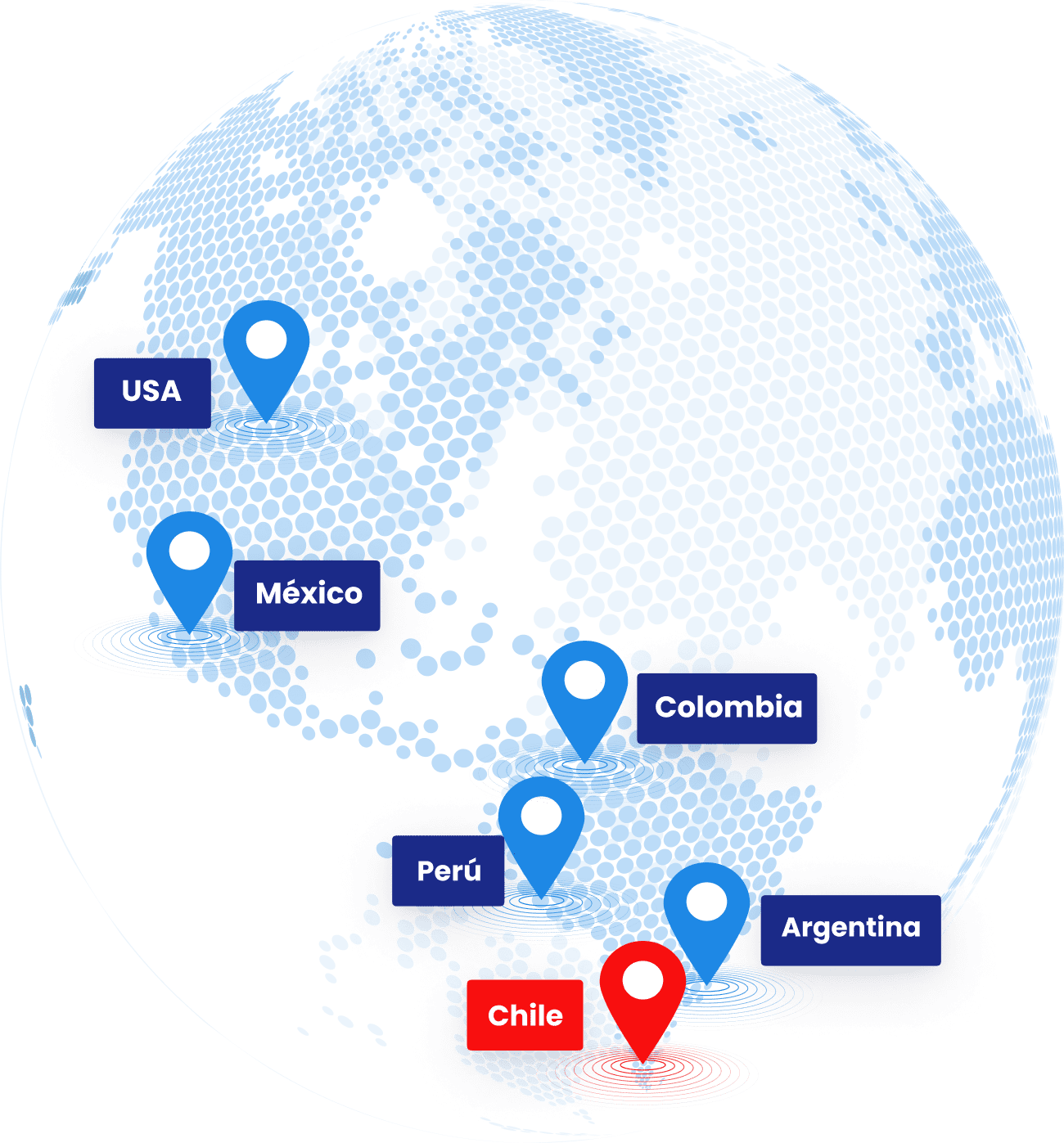
We offer global presence through our strategic partnerships with local players
North America
USA: CRO Partner
Mexico: CRO Partner
South America
Chile: Headquarters (Santiago)
Argentina: CRO Partner
Peru: CRO Partner
Colombia: CRO Partner
Chile is an excellent location for clinical research
Chile is a hub of innovation and research in Latin America given its competitive advantages in clinical research
- Research centers of excellence with extensive experience in academia that generate international trust
- Clinical research culture due to more than 30 years of experience developing clinical studies
- Globally competitive regulation
- Scientific ethics committees accredited by international standards
- World-class healthcare infrastructure
- Wide network of experienced researchers
Get to know our CEO
BOPAL was founded in 2019 by Pablo García, given his passion for clinical research, personal growth, entrepreneurship, innovation, technology and designing creative business models to solve problems.
With more than 13 years of experience in the international pharmaceutical industry -in clinical research, pharmaceuticals, medical devices and commercial areas- Pablo is a regulatory expert, pharmacist graduated from the Pontificia Universidad Católica de Chile, Master in Marketing and Commercial Management from the Universidad
Adolfo Ibáñez.
Former Regulatory Manager of the Clinical Studies Unit in Sanofi Chile.
Pablo is BOPAL's CEO
















